Which Unit Can Be Used to Express Solution Concentration
Up to 24 cash back A kelvins B milliliters C joules per kilogram Dmoles per liter 3The concentration of a solution can be expressed in A 100 B200 C 0500 D 400 4What is the molarity of a solution of KNO3. The answer choices were.

Lesson Explainer Molar Concentrations Nagwa
Which unit can be used to express the concentration of a solution.

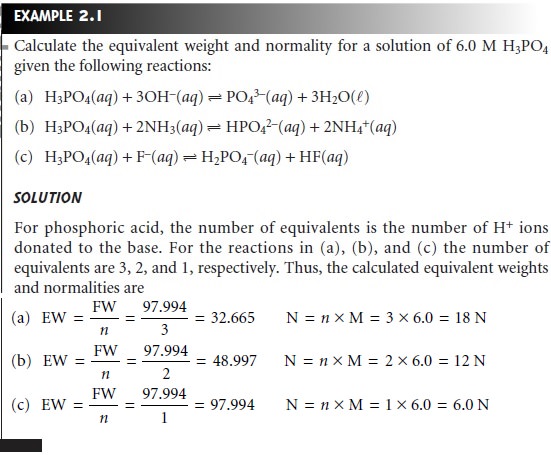
. The massvolume percent is used to express the concentration of a solution when the mass of the solute and volume of the solution is given. Thus if one gram molecule of a solute is present in 1 kg of the solvent the concentration of solutions is said to be one molal. 1 Jmol 3 molL 2 Lmol 4 mols.
Parts per million ppm is used to express the concentration when a very small quantity of solute is present in a large quantity of the solution. Percentage however it is often used as an easy concentration unit since volumes of. Concentration of solution Solute mass in gram Solution volume in liters.
A0005 g B005g C05 g D5g 8What is the total mass of solute in 1000. Concentration amount solute part amount solvent whole Chemists can express concentrations in various ways including. Molality is the most convenient method to express the concentration of solutions because it involves the mass of liquids rather than their volumes.
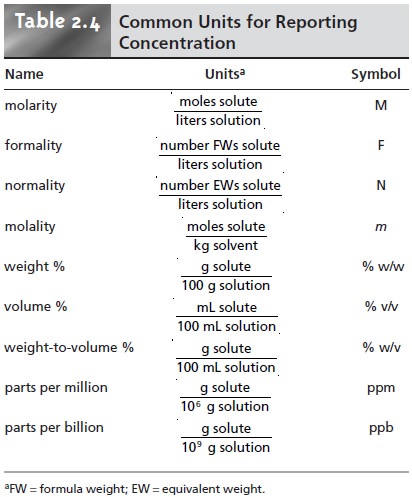
Molarity M indicates the number of moles of solute per liter of solution molesLiter and is one of the most common units used to measure the concentration of a solution. Since the numerator and denominator have different units this concentration unit is not a true relative unit eg. It can be expressed by the following formula.
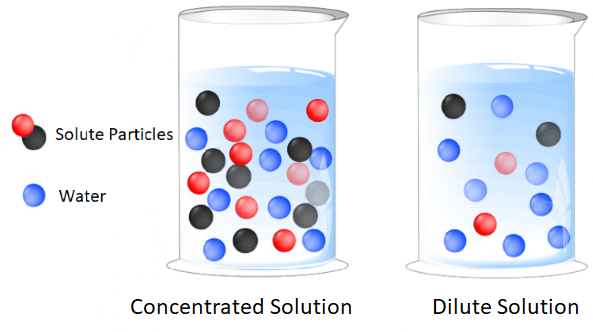
The volume or weight concentration of some solute is expressed in the percentage. A solution can be qualitatively described as. 4 Ways to Express Concentrations in Solutions If you were drinking Kool-Aid you might describe the solution as dilute or concentrated as a means to express concentration.
There are Five main ways we describe the concentration of solutions. And 5 Parts Per Million or Billion. A 35 g B35 M C 35 mL D 35 mol 2Which expression could represent the concentration of a solution.
Report an issue. Which unit can be used to express solution concentration. Which unit can be used to express the concentration of a solution.
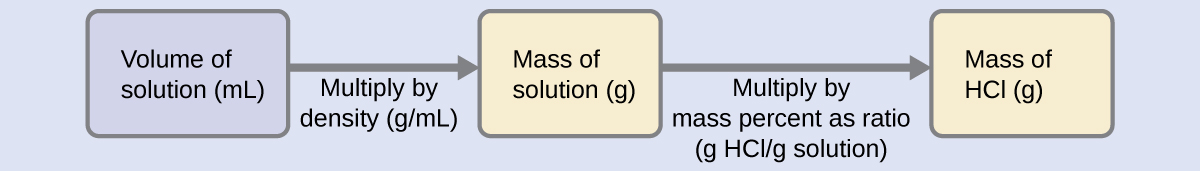
For example 10 gin of sodium chloride is added in. You should know the meaning of each of these terms and more importantly how to convert from one to the other. Which unit can be used to express solution concentration.
Correct answer - Which unit can be used to express the concentration of a solution. 3 question Which unit can be used to express solution concentration. The result can be expressed as mass percentage parts per million ppm or parts per billion ppb.
KPa is used to express pressure. Jg is used to express energy per unit mass. The correct answer is ppm or parts per million.
Suppose a solution of ethanol is marked 025 M this means that in one litre of the given solution 025 moles of ethanol is dissolved. Very low solute concentrations are often expressed using appropriately small units such as parts per million ppm or parts per billion ppb. The concentration of a solution is a macroscopic property represents the amount of solute dissolved in a unit amount of solvent or of solution and can be expressed in a variety of ways qualitatively and quantitatively.
Qualitative Expressions of Concentration. Up to 24 cash back 7Which unit can be used to express the concentration of a solution. The concentration of a solution is expressed by following methods.
Strength or Solution Concentration Concentration in Gram per Liter. 1342 mass percentage mass of solute mass of solution 100. Molarity can be used to calculate the volume of solvent or the amount of solute.
It is the number of moles of solute dissolved in one litre of a solution. It is also independent of the variation in temperature. Another common way of describing concentration is as the ratio of the mass of the solute to the total mass of the solution.
Like percentage part per hundred units ppm and ppb may be defined in terms of masses volumes or mixed mass-volume units. Parts per Million and Parts per Billion. It is defined as the mass of solute present in one milion parts by mass of the solution.
Molecular mass 101 that contains 404 grams of KNO3in 200 liters of solution. Grams of a solution having a concentration of 5 parts per million. 1 ls 3 ppm 2 jg 4 kpa.
A Ls bJg cppm and dkPa. Molarity M Parts per million ppm. This term can be defined as the amount of the solute mass in gram present in one liter of the solution.
I think you were looking for the answer to the chemistry castelearning question. A08 ppm B8 ppm C80 ppm D800 ppm 9What is the concentration of O2g in parts per million in a solution that contains 0008 gram. Concentration is a general measurement unit stating the amount of solute present in a known amount of solution Units for Expressing Concentration Although the terms solute and solution are often associated with liquid sam- ples they can be extended to gas-phase and solid-phase samples as well.
Ls is used to express flow rate. Percentage concentrations based on the solution components masses volumes or both are useful for expressing relatively high concentrations whereas lower concentrations are conveniently expressed using ppm or ppb units. One of the most commonly used methods for expressing the concentrations is molarity.
In addition to molarity a number of other solution concentration units are used in various applications. The unit of molarity is mol kg-1. SI units of expressing concentration of solutions.
A grams of NaCl per liter of water B grams of NaCl per liter of solution C moles of NaCl per liter of water Dmoles of NaCl per liter of solution 3The molarity of an aqueous solution of NaCl is defined as the A Jmol B Lmol CmolL D mols. So the unit of this term is gram per liter.

Solution Concentration Ppt Video Online Download
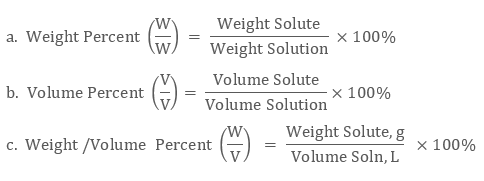
Expressing Concentration Of Solutions Study Material For Iit Jee Askiitians
3 4 Other Units For Solution Concentrations Chemistry
Units For Expressing Concentration

Concentration Of A Solution Geeksforgeeks
Units For Expressing Concentration

Solution Concentration Chemistry Master
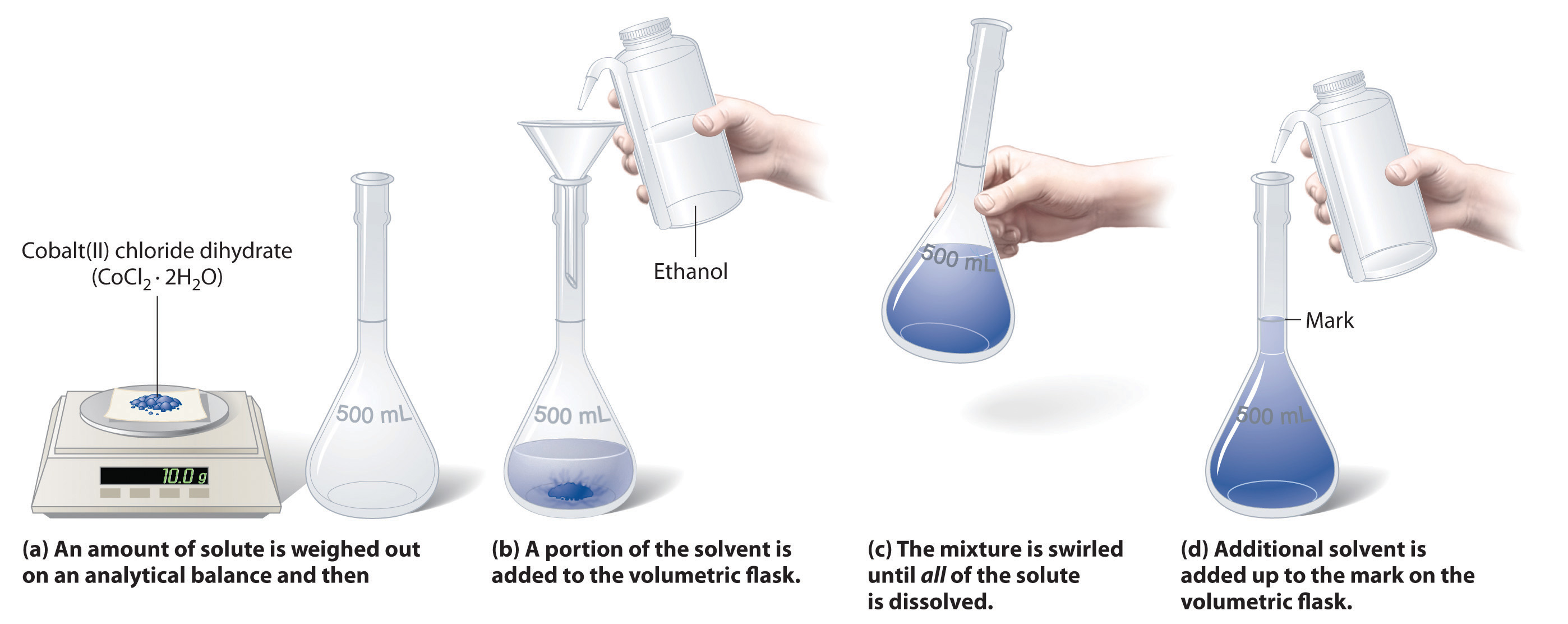
5 2 Solutions And Dilutions Chemistry Libretexts

Solution Concentration Chemistry Ways To Express Solution Composition Molarity Mass Volume Parts Per Million Ppm Ppt Download

Ch104 Chapter 7 Solutions Chemistry

13 2 Solution Concentration Chemistry Libretexts

How To Calculate Solution Concentration In Molarity And Percent By Mass

Expressing Concentration Of Solutions Methods Formulas Videos Q A

Solutions And Concentration Units Of Solutions Molarity Mole Fraction Molarity Formula And More

Concentration Of A Solution Geeksforgeeks

8 1 Concentrations Of Solutions Chemistry Libretexts
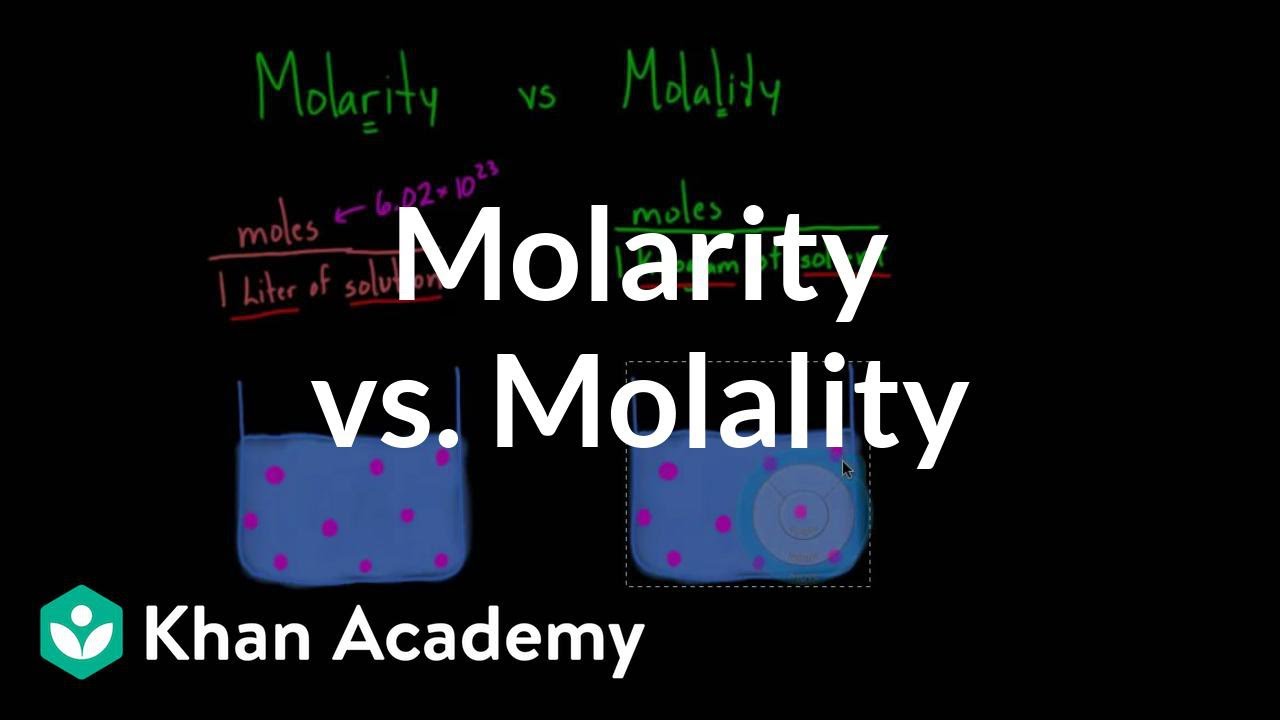
Concentration Units Chemistry Master